This post was written by Annie Lincoln, Ph.D. Student within the Cancer Prevention Group at King’s College London. Annie is interested in improved colorectal cancer surveillance for individuals with a hereditary predisposition to cancer, namely in the context of Lynch Syndrome.
An emergency service for patients with Lynch Syndrome:
In the wake of the COVID-19 pandemic this time last year, a majority of clinical appointments were cancelled throughout the United Kingdom. While most of these cancelled clinical appointments were considered to be “non-urgent”, they included important clinical visits for patients with cancer, or screening and surveillance appointments for those at high risk of developing specific cancers. One such group of patients who were directly impacted by the sudden cancellation of their routine surveillance appointments were individuals with Lynch Syndrome.
Lynch Syndrome is an inherited cancer predisposition syndrome which is characterised by the presence of pathogenic or germline mutations within any one of the mismatch repair (MMR) genes. Though Lynch Syndrome is a rare disease, it affects 1 in 125 individuals within the UK and poses a high lifetime risk of bowel cancer. Depending on the MMR mutation, age, and gender, bowel cancer risk may vary anywhere between 10% to 47% in this patient population. In considering their elevated lifetime risk of bowel cancer, current national clinical guidelines advise patients with Lynch to undergo routine 2-yearly colonoscopy for prevention and surveillance.
In light of the suspension of these services, myself, Professor Sasieni and Dr Kevin Monahan together with other clinical colleagues throughout England, introduced an emergency pathway which enabled the delivery of at-home self-sampling diagnostic devices, known as faecal immunochemical testing (FIT) kits, with priority colonoscopy for those who tested positive.
Addressing an urgent need when existing resources are strained.
In April 2020, just a couple of weeks after the country commenced its initial lockdown, the British Society of Gastroenterology (BSG) and the Joint Advisory Group (JAG), released an announcement, which advised the immediate suspension of endoscopy procedures for non-urgent or routine screening patient populations. Like most clinical appointments in the early days of the pandemic, certain exceptions were stipulated for patients who may require emergency intervention. As such, BSG and JAG provided clear guidance in the expedited triaging of colonoscopy procedures for individuals who may be exhibiting bowel cancer symptoms, and/or those who were found to have a positive test on FIT.
Though this early guidance was certainly justified at a time when most NHS clinical staff and resources were being diverted to emergency efforts surrounding the COVID-19 pandemic, the management of patients requiring routine surveillance for gastrointestinal cancers, such as individuals with Lynch Syndrome, was not well defined.
To address this concern, as well as to prevent Lynch Syndrome patients from being indefinitely deferred for colonoscopy should they be misclassified as being “non-urgent”, we developed a nationwide clinical service evaluation which would administer FIT kits to the homes of eligible Lynch Syndrome patients in lieu of their scheduled colonoscopy, the majority of which had been indefinitely postponed at that time.
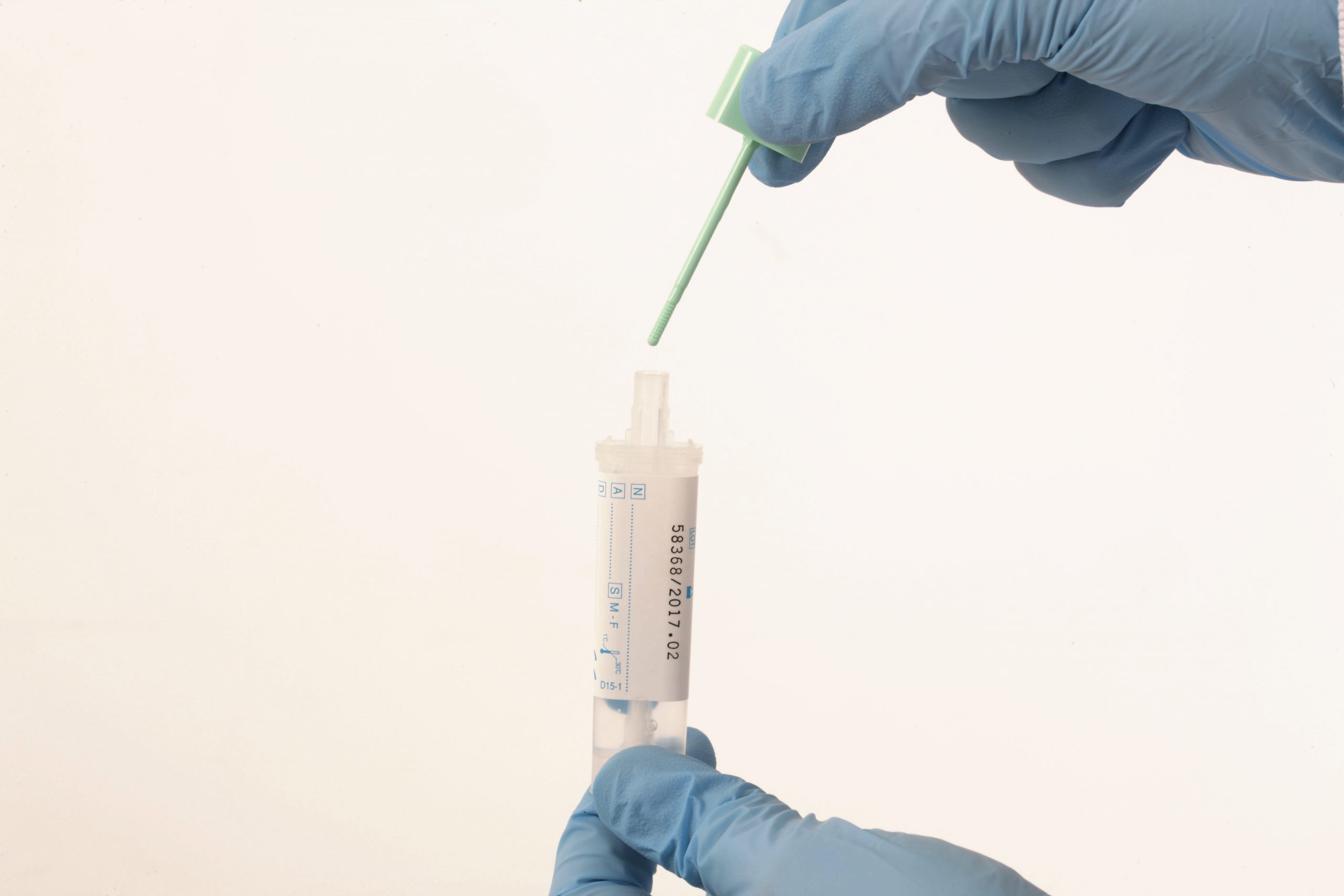
In June 2020 this clinical service was implemented in NHS facilities throughout England. For Lynch patients who had been due for surveillance colonoscopy any time between March 2020 – March 2021, a personalised letter, FIT kit (OC-Sensor™ brand), FIT instructions, and a brief questionnaire was mailed to their home. Patients who were interested in participating would then provide a small stool sample which was collected within the FIT kit and posted back to the NHS Bowel Cancer Screening South of England Hub (Southern Hub) in Surrey. This FIT device, which is routinely used as part of the NHS Bowel Cancer Screening Programme, can detect microscopic traces of blood (haemoglobin) in stool, which may be indicative of bowel cancer. For the purposes of this clinical service, FIT was used to identify the greatest at-risk Lynch patients, which was defined by meeting or exceeding a sensitivity threshold of 10µg of haemoglobin (Hb) per gram of faeces. For the subset of patients who were found to have levels of Hb at or exceeding 10µg, they would then be referred for an emergency colonoscopy via the two-week wait (2WW) pathway.
In addition to a FIT kit, eligible Lynch patients also received a brief 1-page (2-sided) questionnaire which inquired about their overall experience with FIT, and any concerns they may have as it pertains to interruptions made to their bowel cancer surveillance due to the pandemic.
What does the early data show?
Since its implementation in early June 2020, just over 525 FIT kits and questionnaires have been posted to eligible Lynch patients throughout England, with 345 returned to the Southern Hub for analysis. Of the 345 patients who returned their FIT devices, 59 (17%) had faecal Hb (f-Hb) ≥10µg Hb/g faeces and met the criteria for urgent colonoscopy triage via the 2WW pathway. In an early subgroup analysis from this clinical service, 9 of 67 (13%) patients from St. Mark’s Hospital were referred to emergency colonoscopy via the 2-week wait pathway based solely on their positive FIT results.
In addition to the data being collected from these FIT results, as well as available colonoscopy and histology data, an assessment on the attitudes of acceptability towards FIT as a novel intervention in this patient population is being conducted from qualitative data that will be gleaned from the patient questionnaires.
Though data analysis is on-going and is pending the conclusion of the clinical service at the end of March, the preliminary data is suggestive of FIT as an effective mechanism in efficient triage in this high-risk patient population when staff and resources are strained.
What’s Next?
Though this clinical service was implemented as an emergency measure at the height of the COVID-19 pandemic, the utility of FIT in patients with Lynch Syndrome warrants further investigation. Should FIT prove to be an effective method of surveillance in this patient population, the benefits to patients and clinical staff could be quite promising, including the potential for less costly and invasive colonoscopy procedures, thereby alleviating pressure from strained NHS staff and resources. Moreover, FIT may have the potential to mitigate added burdens and pressures from patients who may otherwise be unable to attend regular clinical visits.
As part of my PhD thesis, I aim to examine the efficacy of FIT in the Lynch Syndrome patient population in greater detail, and I am currently in the process of developing a multi-Institutional pilot research study along with Dr Kevin Monahan, Chief Investigator (CI), Prof. Peter Sasieni, Co-Investigator, and other clinical colleagues, which has incorporated early insights from the clinical service in creating the overall study design. Following a recent Ethics review, the team of physicians and researchers hope to begin patient recruitment later this year.
The views expressed are those of the author. Posting of the blog does not signify that the Cancer Prevention Group endorse those views or opinions.
Share this Page

Leave a Reply