As 2024 draws to a close, we’re reflecting on an incredible year for our team. Since formally moving to QMUL at the end of 2023 and joining forces with colleagues here to become the Centre for Cancer Screening Prevention and Early Diagnosis, we’ve thrived in our new environment – building collaborations, driving cutting-edge research, and delivering on our mission to prevent and detect cancer earlier.
Here’s a look back at some of our biggest successes of the year!
Advancing Self-Testing for Cervical Screening
YouScreen: Bringing Screening to More People
In 2021, we ran the YouScreen study, offering HPV self-sampling kits to non-attenders via GP visits and direct mail. Published this year, the results showed that these easy-to-use kits could significantly expand cervical screening access. YouScreen was the largest UK trial of its kind and the first to integrate HPV self-sampling into the NHS cervical screening programme.
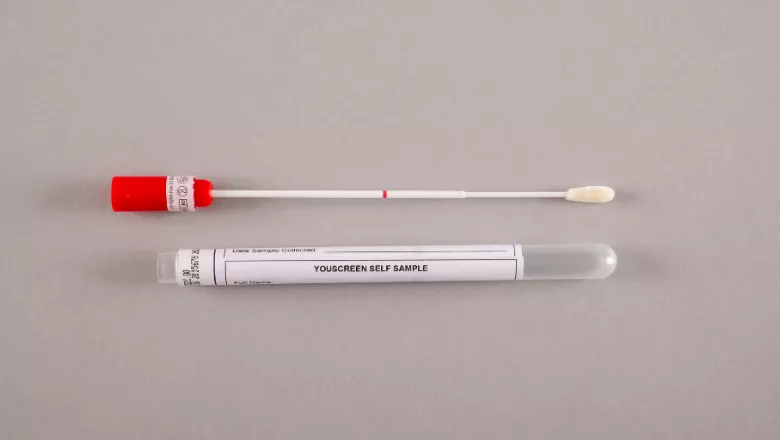
Tailoring Clinical Pathways
Building on YouScreen, we explored risk-based triage using HPV type and cycle threshold (Ct) values from self-samples. By identifying high-risk individuals – those with a 43% likelihood of CIN2+ – this approach could streamline clinical management and reduce unnecessary procedures. The study, published in PLOSMedicine, highlights the importance of refining self-sample triage processes through further prospective research. Optimising workflows and follow-up protocols could make HPV self-sampling a practical, targeted approach to improving cervical cancer prevention and closing screening gaps.
You can read the QMUL story here.
HPValidate: Accuracy and Acceptability
We recently published results from another UK study, HPValidate, a study to explore the accuracy of HPV self-sampling compared to clinician-collected tests. Results showed that four self-sample test combinations are ready for consideration in the UK’s National Cervical Screening Programme.
We also looked at the acceptability of self-testing among women using the test with 98% of participants reporting a positive experience.
In addition, we learned that while many women want the choice of self-sampling (85%), a clear recommendation is also desired. Researchers believe these findings highlight an urgent need to explore and evaluate different methods of offering this choice, ensuring that effective messaging is used during any implementation or assessment of HPV self-sampling options in the UK and beyond.
These findings have informed the UK National Screening Committee’s call for self-sampling to be implemented nationwide.
HPV Vaccination
Our ongoing work looking at the impact of the HPV vaccination programme has been a huge success. Most recently we have completed a project using population data from 2006 to 2020, which was published in the BMJ, demonstrates just how far-reaching its effects have been. High coverage of routine HPV vaccination among girls aged 12–13 has led to a remarkable reduction in cervical disease rates, with the benefits most pronounced among women in deprived areas who have historically faced the highest burden of disease. Not only has the vaccine substantially lowered the rate of cervical cancer across the population, but it has also played a critical role in reducing health inequalities, a major step forward in health equity. However, despite these successes, cervical disease rates in deprived groups remain higher than in less deprived populations, highlighting the need for continued action
The study suggests valuing healthcare gains in deprived populations more highly, in line with societal preferences and existing health economic approaches. Greater investment in reaching underserved groups could amplify the vaccine’s impact, further narrowing inequalities and advancing WHO’s cervical cancer elimination targets.
Revolutionising Early Detection
Multi-Cancer Early Detection (MCED)
Imagine a single blood test that could detect multiple cancers before symptoms even appear – this is the groundbreaking potential of Multi-Cancer Early Detection (MCED) testing. In 2024, we completed the AMPS project, exploring the acceptability of MCED tests for population screening. This work included a qualitative component and a population-based survey. Our findings showed that while most people (over 90%) are keen to take an MCED test, concerns remain around test accuracy and false positive result. Interestingly, socio-demographic factors (like age, sex and deprivation) were not associated with wanting the test – however it is likely that, as for other screening programmes, there will be inequalities in uptake if MCED screening is rolled out. It will therefore be essential to monitor and address inequalities to ensure these innovations benefit everyone.
Watch out for a dedicated blog series on MCED testing in 2025!
NHS-Galleri: A Milestone in Cancer Detection
In summer 2024, we celebrated the final follow-up visits for our NHS-Galleri trial, a trial delivered by our Cancer Research UK Cancer Prevention Trials Unit, exploring how blood-based early detection tests can transform cancer screening. With 140,000 participants , this was a huge milestone, and you can see the video marking the end of appointments and celebrating the contributions of everyone involved.
Stay tuned for results in 2026!
YORKSURe: Pioneering Bladder Health Screening
In 2024, we concluded the YORKSURe trial – a feasibility study funded by Yorkshire Cancer Research exploring urine self-testing and community clinics for bladder health issues. Results are in preparation, and we’re excited to share findings soon!

BEST4: Screening and Surveillance for Oesophageal Cancer
BEST4 is programme of research looking at the efficacy of the capsule sponge (often known as the Cytosponge) in detecting and monitoring Barrett’s Oesophagus. This condition is a known precursor to oesophageal cancer. BEST4 is focused on gathering robust data to evaluate whether the capsule sponge could be used as a surveillance tool for people diagnosed with Barrett’s Oesophagus to improve outcomes for oesophageal cancer at a population level.
The capsule sponge starts off as a small, coated pill attached to a thread. When swallowed, the coating dissolves releasing a small sponge which collects cells from the lining of the oesophagus as it is retrieved. These cells can then be analysed for changes indicative of Barrett’s Oesophagus or early signs of cancer.
Cancer Research UK funded previous clinical trials demonstrating the test is safe and accurate. The BEST3 trial, showed the test picks up 10 times more cases of Barrett’s oesophagus in people with chronic heartburn, compared to endoscopy.
2024 was a year of beginnings for the BEST4 trials.
- BEST4 Surveillance recruited its first participant, testing the capsule sponge as an alternative to endoscopy for monitoring Barrett’s Oesophagus. The condition, a precursor condition to oesophageal cancer, is where cells in the food pipe start to grow abnormally.
- BEST4 Screening trial launched in November, recruitment of individuals who regularly take medication for heartburn has started and the team are already seeing people in the mobile vans. The recruitment target is 120,000 over the next 3 years. Heartburn is the most common symptom for Barrett’s Oesophagus. Invitations to join the trial will be sent by text message from NHSResearch. Participants will be asked to the platform called Heartburn Health. The platform enables people to take part in clinical trials looking at cancers linked to heartburn. Several thousands of people have already signed up.

These trials have the potential to transform how we approach the early detection of oesophageal cancer, enabling earlier diagnosis and treatment while reducing the reliance on more invasive procedures like endoscopy.
New NICE guideline for ovarian cancer
Each year, around 7,500 women are diagnosed with ovarian cancer. What many don’t realise is that about 1 in 5 of these cases are linked to high-risk genes. These inherited genetic changes, or pathogenic variants, significantly increase the risk of ovarian cancer. Yet, many people carrying these variants remain unaware of their risk.
We are proud that earlier this year, years of hard work and successful research was recognised via publication of NICE guidelines for ovarian cancer. These new guidelines have helped shine a light on the importance of genetic testing – not just for women, but for anyone who may carry and pass on these variants. This includes women, men, trans, and non-binary individuals alike, as these genetic alterations don’t discriminate by gender identity or assigned sex at birth. For example some genetic variants also increase risk of prostate cancer. A man may have passed on the faulty gene to his daughters.
By raising awareness about genetic testing, we’re giving more people the tools to take charge of their health. Preventive measures, like risk-reducing surgeries, mean fewer people go on to develop ovarian cancer. Additionally, this research has been incorporated into guidelines broadening access and personalising risk-reducing surgery for women at increased risk.
Check out this interview with the lead Professor: 5 mins with Ranjit Manchanda
NHS Jewish BRCA Programme
The NHS Jewish BRCA Programme, launched in January 2024, is an initiative set to transform cancer prevention and early detection for individuals of Jewish ancestry by identifying thousands at increased risk for certain cancers.
The programme offers a simple and accessible saliva test to anyone with at least one Jewish grandparent, allowing them to find out if they carry the BRCA1 or BRCA2 gene mutations. These mutations significantly increase the risk of breast and ovarian cancer, and in the case of BRCA2, prostate cancer.
As part of its national roll-out, the programme aims to test approximately 30,000 people over the next two years, empowering individuals and families with potentially life-saving information and access to early interventions. This marks a significant step forward in personalized cancer prevention and care.
Highlights from the 3rd AWACAN-ED School
AWACAN-ED (African aWAreness of CANcer & Early Diagnosis) is an NIHR-funded Global Health Research Group focussing on Advancing Early Diagnosis of Cancer in Southern Africa. Building on the success of the first two Schools, the 3rd AWACAN-ED School gave scholars an invaluable platform to showcase the progress of their research projects, receive expert feedback from AWACAN-ED’s Principal and Co-investigators, and fine-tune their study designs and methodologies. From insightful workshops to real-world challenges, this year’s AWACAN-ED School was a perfect blend of inspiration, learning, and collaboration – pushing scholars closer to their goals in advancing cancer research.

One of the standout moments of the programme was an engaging and hands-on session, exploring the impact of intersectional discrimination in cancer research. In small groups, participants worked through real-world cancer-patient case studies, identifying potential biases, discriminatory attitudes, and inappropriate management practices. The session closed with a dynamic discussion on career development pathways, sharing experiences, challenges, and aspirations for the future – a truly motivating way to wrap up the School!
You can read more about the School in this blog post by Jone Garcia Lurgain
Fostering Collaboration and Inclusion
Our successes this year have been shaped by collaboration, a deep commitment to Equality, Diversity and Inclusion (EDI), and meaningful Patient and Public Involvement (PPI). By actively engaging patients and communities, we are ensuring our research is both inclusive and also reflective of the voices and needs of those it aims to serve. These collaborations have driven innovation and strengthened our projects, and we look forward to advancing this work over the next year.
- Equality, Diversity and Inclusion (EDI): We have made significant strides in our EDI efforts this year, including the launch of the DISTINCT initiative, which aims to increase the diversity of participants in clinical trials. Working alongside The Clinical Trials and Statistics Unit at the ICR (ICR-CTSU), the initiative is now being piloted.
- PPI at the Heart: Patient and Public Involvement (PPI) remains central to our work. Our PPI teams have also been instrumental in ensuring that our research remains patient-centred, with numerous PPI panel workshops and feedback sessions held throughout the year. These sessions tackled strategic topics such as integrating PPI at QMUL, data control, and driving innovation in PPI. Beyond strategy, PPI plays a vital role in individual trial processes, where representatives actively contribute to the planning, design, and execution of trials, ensuring patient voices are heard at every stage.
A Heartfelt Thank You
Our achievements in 2024 would not have been possible without the unwavering commitment of our incredible team and partners:

- Our PPI representatives who are willing and able at every stage to ensure that we are keeping patients and the public at the heart of everything we do
- Our dedicated nurses and secondary care teams whose exceptional efforts drive the delivery of both research and high-quality care to our trial participants.
- Our chief investigators, sponsors and funders who have the passion and drive to support us and enable our success.
- Collaborative partners who have been crucial in delivering aspects of our decentralised trials, making research more accessible and inclusive for participants.
- And most importantly, our tens of thousands of trial participants – your generosity and trust are the foundation of everything we achieve, and we are proud to have you as part of our team.
Looking Ahead to 2025
As we step into the new year, we’re excited to embark on new projects, adapt to evolving clinical trial regulations, and continue making strides in cancer screening, prevention and early diagnosis. Together, we will ensure our research remains cutting-edge, inclusive, and impactful.

