This post was written by Chris Deeley. Chris is the Trial Coordinator for the BEST4 Screening trial, managed by the Cancer Prevention Trials Unit (CPTU) within the Centre for Cancer Screening, Prevention and Early Diagnosis, at Queen Mary University of London. Prior to joining the BEST4 team, Chris worked as the Trial Administrator on the NHS-Galleri trial with the CPTU.
.
The BEST4 Surveillance trial has recently opened its doors to patient recruitment, recruiting its first patient – a 49-year-old male from Cambridge – on 9th January 2024. The trial will test an innovative capsule sponge device for early detection and monitoring of oesophageal cancer (cancer of the food pipe). It is hoped this could offer a quicker and less invasive alternative to current diagnostic options.


EndoSign® by Cyted
Oesophageal Cancer Diagnostics: the current picture
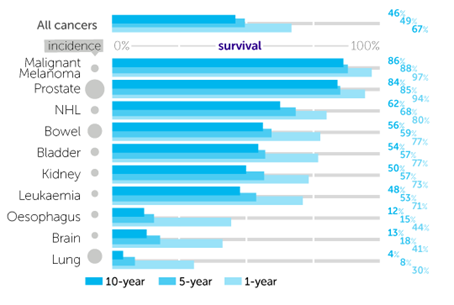
Although not among the 10 most common cancers in the UK, oesophageal cancer ranks as the 7th most deadly cancer. It is estimated that 8,000 people will die in the UK each year from the disease, with only 15% living more than 5 years after diagnosis.
Credit: Cancer Research UK
People with Barrett’s Oesophagus are known to be at higher risk of developing oesophageal cancer. This is a condition in which abnormal, pre-cancerous cells are found in the oesophagus (food pipe). Individuals with experience of heartburn, indigestion and acid reflux are more likely to develop Barrett’s Oesophagus.
At present, Barrett’s Oesophagus patients are monitored via regular hospital endoscopies. Endoscopy involves guiding a small camera, attached to a thin tube, down the oesophagus. A biopsy (sample) of cells is then taken from the oesophagus, for subsequent laboratory testing.
While endoscopies are a vital tool in diagnosing cancer, they can be highly uncomfortable for patients. In turn, they cannot be performed without a specialist. This has led to long hospital waiting lists, coupled with high financial costs to the NHS.
This could soon change thanks to the revolutionary capsule sponge device.
‘Pill on a Thread’
The device, also known as a ‘pill on a thread’, works by swallowing a capsule attached to a thread. The capsule casing dissolves upon reaching the stomach, releasing a small spherical sponge. As the thread is pulled back out from the oesophagus, by a specially trained nurse, the sponge collects large quantities of oesophageal tract cells. These cells – along with the sponge – are then sent to a specialist laboratory for testing.
BEST4 Trial: Surveillance Trial + Screening Trial
The BEST4 trial is comprised of two separate components: the BEST4 Surveillance Trial and the BEST4 Screening Trial:
- The BEST4 Surveillance Trial will look at whether the capsule sponge can effectively be used in place of endoscopy, to monitor patients with Barrett’s Oesophagus.
- The BEST4 Screening Trial, set to launch in summer 2024, will investigate the capsule sponge’s effectiveness as a screening tool for Barrett’s Oesophagus. This will look at people who suffer from heartburn, indigestion or acid reflux.
BEST4: building on previous clinical trial success
The capsule sponge has been designed, and further refined, by Professor Rebecca Fitzgerald and her team at the Cancer Research UK Cambridge Centre and Early Cancer Institute at the University of Cambridge. Previous trials – including BEST1, BEST2 and BEST3 – have successfully shown that the capsule sponge device is safe and accurate. It is able to detect up to 10 times more cases of Barrett’s Oesophagus compared with standard practice.
Dr Iain Foulkes, Executive Director of Research and Innovation at Cancer Research UK, said: “Around 59% of all oesophageal cancer cases are preventable. Yet endoscopy, the gold standard for diagnosing and treating this cancer, is labour-intensive. We need better tools and tests to monitor people most at risk.
“Backed by funding from Cancer Research UK, the capsule sponge has become one of the most exciting early detection tools to emerge in recent years. It’s a remarkable invention by Professor Fitzgerald and her team.
“There are 9,200 people diagnosed with oesophageal cancer in the UK every year. The capsule sponge will mean they can benefit from kinder treatment options, if their cancer is caught at a much earlier stage.”
BEST4: what’s next for patients?
With the BEST4 Surveillance Trial having opened in Addenbrooke’s Hospital, Cambridge, it is set to extend to 11 additional hospitals across England. It intends to enrol 2,000 patients in total. This recruitment will run until September 2025.
The first enrolled patient – Tim Cowper – has to date attended his hospital for routine endoscopy checks every three years. As part of the BEST4 Surveillance Trial, he will now also have a capsule sponge test, prior to his endoscopy. He will be seen for follow-up visits over the next 3 years. Results of his initial capsule sponge test will determine the frequency of these follow ups.
If trial results show the capsule sponge is successful in effectively finding early abnormal cell changes, the device could be used for routine clinical monitoring of Barrett’s Oesophagus patients in the future. This would mean a reduced need for endoscopies in some patients.
This is an exciting prospect. Tim says of the possible new monitoring device: “swallowing a capsule sponge is a much better experience.”

Tim Cowper (49) became the first patient enrolled in the BEST4 Surveillance study
BEST4: what’s next for trial teams?
The second trial, the BEST4 Screening Trial, is scheduled to launch in summer 2024. It will aim to recruit 120,000 individuals on long-term treatment for heartburn, aged between 55 and 79. The trial will be conducted in the community using mobile clinical units. Trial results will determine if the capsule sponge device can be used as a screening tool for detecting Barrett’s Oesophagus, and also for reducing cases of oesophageal cancer diagnosed at a later stage.
.
The views expressed are those of the author. Posting of the blog does not signify that the Cancer Prevention Group endorses those views or opinions.
