Epidemiology, by Dr Emilia Lim |
This is the second in a special four-part series, looking at how air pollution can cause lung cancer in people who have never smoked.
Authors on the paper, Professor James DeGregori, Dr William Hill, and Dr Emilia Lim all contributed to the series.
EGFR-driven lung cancer in never-smokers
As Professor DeGregori mentioned in our last blog post, our motivation for this work stemmed from our interest in understanding how lung cancer develops in never-smokers. We knew that lung tumours in never-smokers frequently harboured mutations in the EGFR gene and had relatively few mutations compared with lung tumours from smokers. Alongside this, they did not appear to have any DNA imprints caused by carcinogens.
We therefore wondered how these cancers were developing in the absence of large numbers of mutations. Could air pollution play a role? If so, how?
Association between PM2.5 levels and incidence of EGFR-driven lung cancer
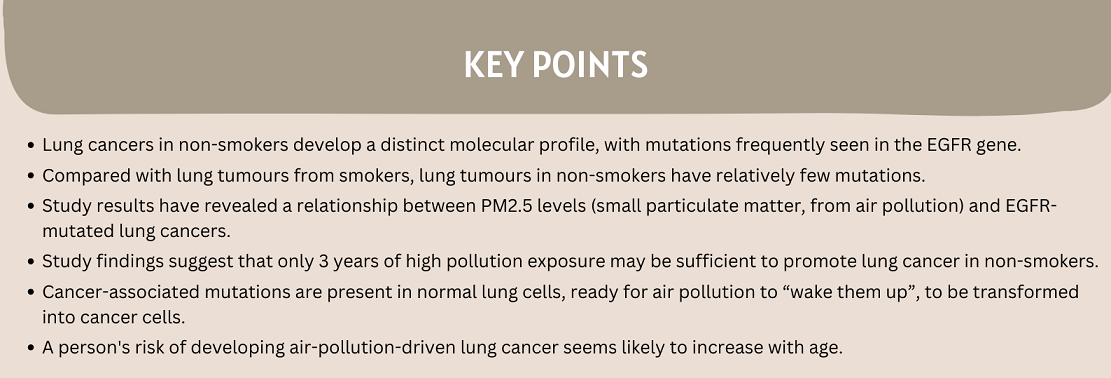
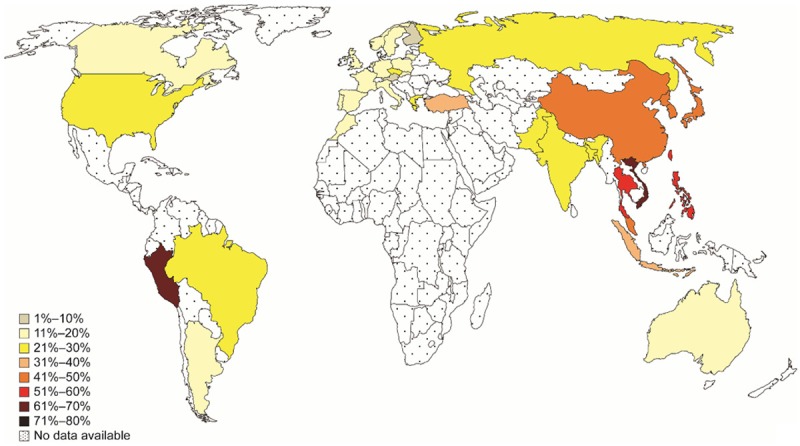
Our collaborator, Dr. Allen Feng-Che Kuan from the Chang Gung Memorial Hospital in Taiwan, brought to our attention a world map of EGFR mutation frequencies in lung cancers. He highlighted how the shading gradients on this map resembled those from the WHO world map of PM2.5 air pollution. This led us to wonder if PM2.5 levels might be associated with EGFR-driven lung cancers.
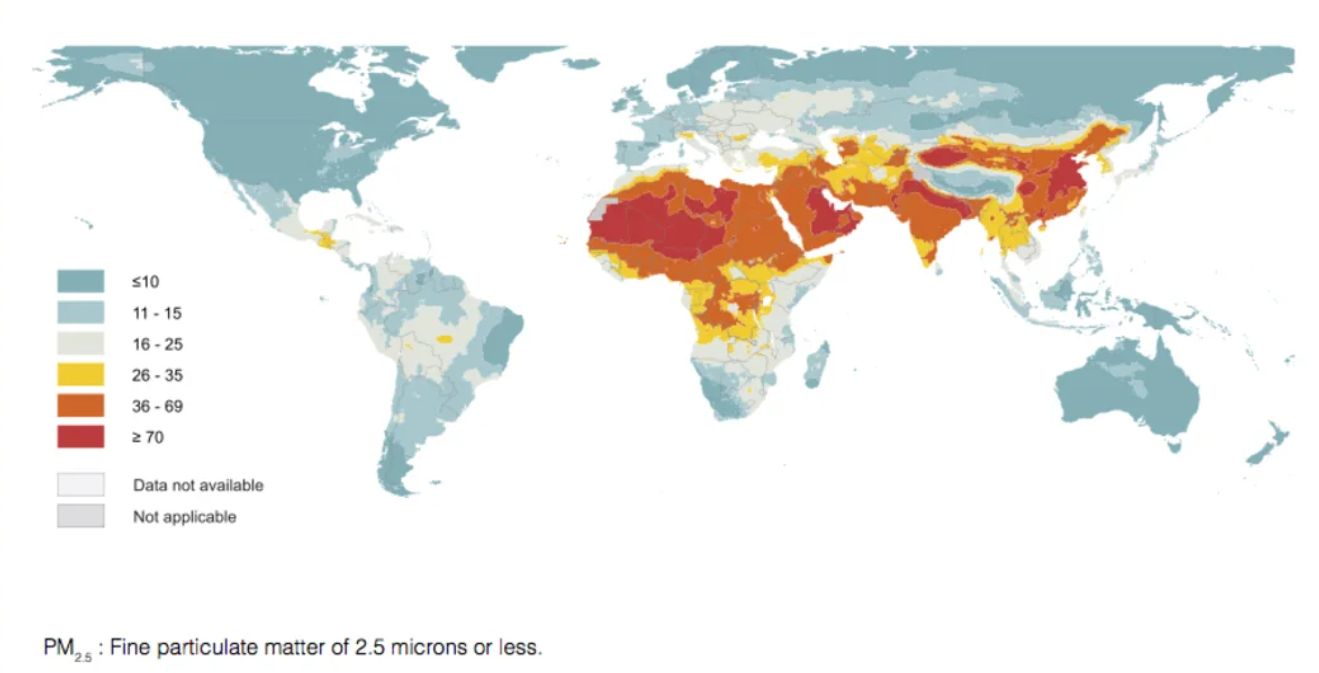
To explore this in England, we began a partnership with Public Health England (now NHS Digital). This was with the aim of relating EGFR-driven lung cancer incidence with mean PM2.5 levels, across the 20 Cancer Alliance regions in England.
We also wanted to include Asian cohorts in our analyses: we wished to examine this trend in other ethnic groups, and in geographical regions with relatively higher PM2.5 levels. Allen therefore performed the same analysis from his data sets in Taiwan. Our collaborators from Samsung Medical Center in South Korea did the same.
Ecological analyses from all three countries revealed that there was indeed a relationship between PM2.5 levels and the incidence of EGFR-mutated lung cancers.
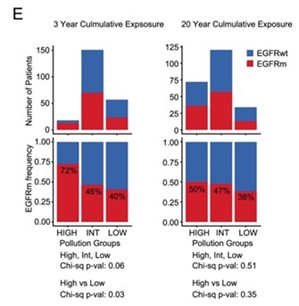
The above analyses were rather crude, in that they only considered one PM2.5 level for each geographical region. Each of these regions could however represent hundreds of patients. We therefore set out to conduct a more refined analysis. We approached Dr. Stephen Lam and Dr. Renelle Myers at BC Cancer Research Institute in Canada, who had a more exhaustive data set. This contained PM2.5 exposure levels for each patient for up to 20 years prior to lung cancer diagnosis.
Analysis of these data revealed that it was 3 years (rather than 20 years) of high cumulative PM2.5 exposure levels that correlated with high EGFR mutation frequency in lung tumours. This suggested that 3 years of high pollution exposure may be sufficient to promote EGFR-driven lung cancer.
Our analysis of the UK Biobank data set was consistent with this. This revealed a relationship between PM2.5 levels and lung cancer incidence in individuals living at the same address for at least 3 years prior to diagnosis.
Credit: Hill, W., Lim, E.L., Weeden, C.E. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159-167 (2023)
Oncogenic EGFR and KRAS driver mutations are common in healthy lung tissue
After observing these significant associations between PM2.5 and EGFR-driven lung cancers, we then wondered how PM2.5 may be causing these cancers to develop. Were these cancer-associated EGFR mutations present in our normal lung cells, ready for air pollution to “wake them up” and transform them into cancer cells?
This was indeed what we found. When we looked at normal lung tissue from individuals both developing and not developing lung cancer in their lifetimes, EGFR mutations were present in up to 18% of samples. While we had initially estimated that they were present in only about in 1 in 600,000 cells, they were there in abundance. And poised for pollution to act on.
Was this limited to EGFR? No! We also observed cancer-associated mutations in KRAS, and in the two dozen other genes we tested. Interestingly, the numbers of mutations present within each never-smoker lung sample seemed to correlate with the patient’s age. This suggested that perhaps our risk of developing pollution-driven cancer could increase as we get older.
Additionally, when examining pollution-associated carbon deposits in the lung tissue, we noted that carbon deposits did not result in more cancer-associated mutations. Instead, these deposits were associated with the multiplication of cells with mutations into large clones with the potential to form cancer.
The next step: using pre-clinical models to further investigate the role of PM2.5
Our findings in patient samples really intrigued us. They inspired us to further tease apart how PM2.5 creates the ideal conditions for cells with pre-existing mutations to rapidly multiply into cancerous clones. For this, we turned to our pre-clinical models and pollution exposure studies.
These are the topic of our next blog post by Dr. William Hill.
The views expressed are those of the author. Posting of the blog does not signify that the Cancer Prevention Group endorses those views or opinions.

