The Introduction, by Professor James DeGregori |
This is the first in a special four-part series, looking at how air pollution can cause lung cancer in people who have never smoked.
Authors on the paper Professor James DeGregori, Dr William Hill, and Dr Emilia Lim all contributed to the series.

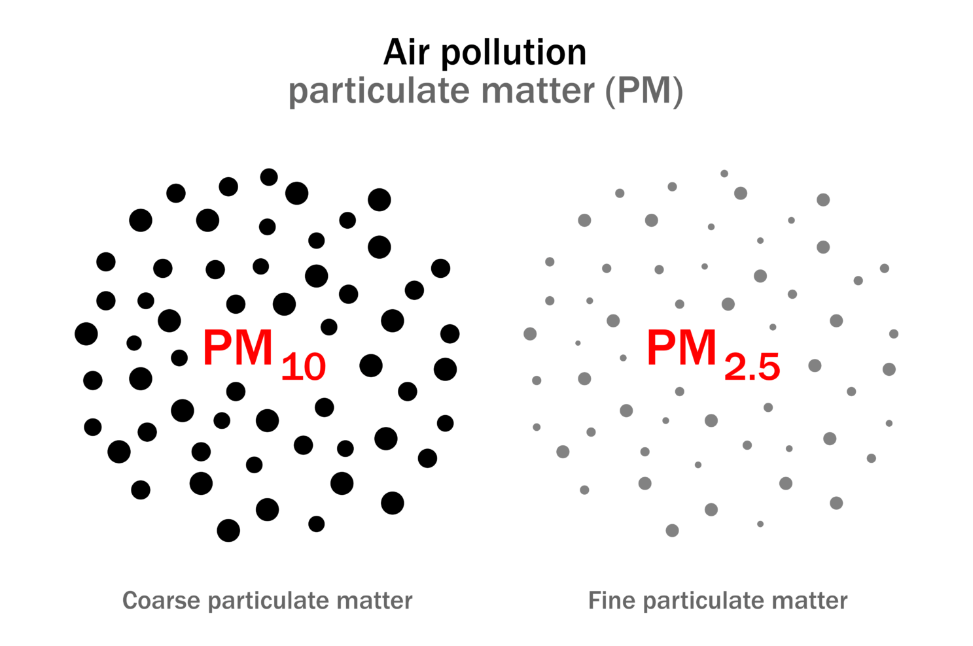
Air pollution and PM2.5
The World Health Organization (WHO) estimates that air pollution kills an estimated 7 million people each year, and accounts for a quarter of a million cases of cancer annually. 99% of the world’s population live in areas that exceed WHO guidelines, with the greatest exposures in low- and middle-income countries. A major culprit implicated in the negative health effects of air pollution is small particulate matter, known as PM2.5: inhalable particles with a diameter of 2.5 micrometres or less.
Lung cancer in never-smokers
The fraction of lung cancers not associated with cigarette smoking has increased over the years, in part due to reduced cigarette smoking in industrialized countries. Studies show a doubling of the fraction of lung cancers in people who never smoked in both the U.S.A. and the U.K. in recent decades. ‘Lung cancers in people who never smoked’ account for up to 20% of all lung cancers, therefore ranking among the top cancers in terms of deaths caused. Thus, understanding the causes of lung cancers in never-smokers is of critical importance.
Oncogenes and tumor suppressors
Mutations in our DNA play a key role in the development of cancers. Some mutations can increase the activity of gene products that can contribute to uncontrolled cell growth and invasive behavior. These mutated genes are known as oncogenes. Alternatively, mutations can disrupt genes that normally control cell behavior and maintain order. These mutated genes are known as tumor suppressors.
Cancers are known to contain a plethora of such mutated oncogenes and tumor suppressor genes, with some of these genes mutated repeatedly in the same cancer types. Lung cancers frequently contain oncogenic mutations in the KRAS and EGFR genes, leading to uncontrolled cell proliferation. EGFR mutations are more common in cancers of people who have never smoked.
Models of carcinogenesis
For decades, explanations for why we develop cancers have been focused on mutation causation. However, more recent studies have revealed key roles of alterations in our tissues resulting from oncogenic ‘insults’. Such insults include radiation, chemotherapy and old age. These contexts alter tissue environments to promote the expansion of clones bearing mutations in oncogenes or tumor suppressor.
It is also notable that these oncogenic insults promote inflammation (activation of the body’s immune system in response to infection or tissue damage, through the release of inflammatory cells). Inflammation has been shown to be critical for promoting selection for these oncogenic mutations. Indeed, a clinical trial of an anti-inflammatory agent (an antibody that blocks interleukin-1β) demonstrated a several-fold reduction in lung cancer.
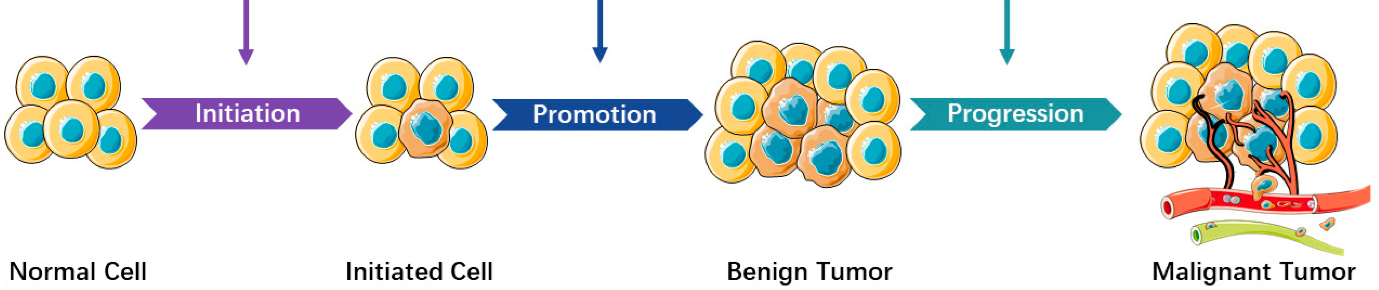
Such results align with the classical “two-step” model of carcinogenesis:
A carcinogen (the initiator) must be followed by a second chemical treatment (the promoter) to result in cancers. The initiator first ‘induces’ mutations, with these pre-existing mutations then selected for through the promoter-mediated impact on tissues.
Increasing understanding of the mechanistic complexities of carcinogenesis
A recent study showed how many known carcinogens do not appear to act by causing mutations (i.e. carcinogens need not be mutagens). Therefore, a key question as applied to air pollution is: does air pollution act as an initiator (inducing mutations) or a promoter (selecting for pre-existing mutations)?
In recent years, there have been significant advances in DNA sequencing technology. These have led to a revolution in our understanding of DNA mutations present in normal (non-cancerous) tissues. We now know that our tissues can often become dominated by small clones, driven by mutations known to contribute to cancers. A typical adult over 60 years old will possess more than 100 billion cells with such cancer-associated mutations (from a total of a few trillion cells).
Some of the mutations driving these small clonal expansions result from environmental exposures, such as cigarette smoking. But many appear to be a consequence of aging. So what keeps these cells with oncogenic mutations from growing into cancers? And what can cause these mutated clones to expand and evolve into life-threatening cancers?
Nature publication

This study – Lung adenocarcinoma promotion by air pollutants – provides valuable insight into how air pollution can promote the evolution of lung cancers from pre-existing oncogenic mutations. It has major implications for how we think about cancer prevention: for research and policy planning alike.
Over the next couple of weeks we will discuss the different stages of the study, together with its results and their implications.
Next up, the epidemiology, by Dr Emilia Lim.
The views expressed are those of the author. Posting of the blog does not signify that the Cancer Prevention Group endorses those views or opinions.

