Cancer screening is never straight forward and can be confusing. We discuss some of the key concepts in cancer screening. You’ll find that we refer to these frequently across our blog posts.
What is cancer screening?
In its simplest definition cancer screening is the use of a test to identify cancer before symptoms arise. Our blog usually discusses population-based screening (also known as mass screening) where a population (usually defined by sex and/or age) is offered the screening test. This should not be confused with selective screening of people known to be at high risk of cancer, for example those known to have genetic mutations that increase the risk of cancer.
Although all cancer screening programmes aim to reduce mortality (the number of people, out of a population of 100,000, dying from cancer in one year) some achieve this by identifying cancers early and others by identifying pre-cancerous lesions and treating them before they become cancer.
For example, cervical cancer screening aims to identify pre-cancerous lesions and hence reduce the incidence (number of cancers diagnosed in 100,000 people in one year) of this cancer. On the other hand, breast cancer screening aims to detect cancers at an early stage, so that they can be treated improving survival. Bowel screening does a bit of both: detecting adenomas before they become cancer but also detecting early stage treatable cancers.
How good are cancer screening tests?
The accuracy of screening test in detecting disease in a population is usually expressed as sensitivity (proportion of people with cancer who will have a positive result on the test) or specificity (the proportion of people with a negative test that do not have cancer). However, the sensitivity and specificity do not tell us the probability that the test will give the correct diagnosis. This is captured by the positive and negative predictive values. The positive predictive value (PPV) of a test is the proportion of people with a positive screening test who will have cancer. The negative predictive value (NPV) of a test is the proportion of people with negative test result who do not have cancer.
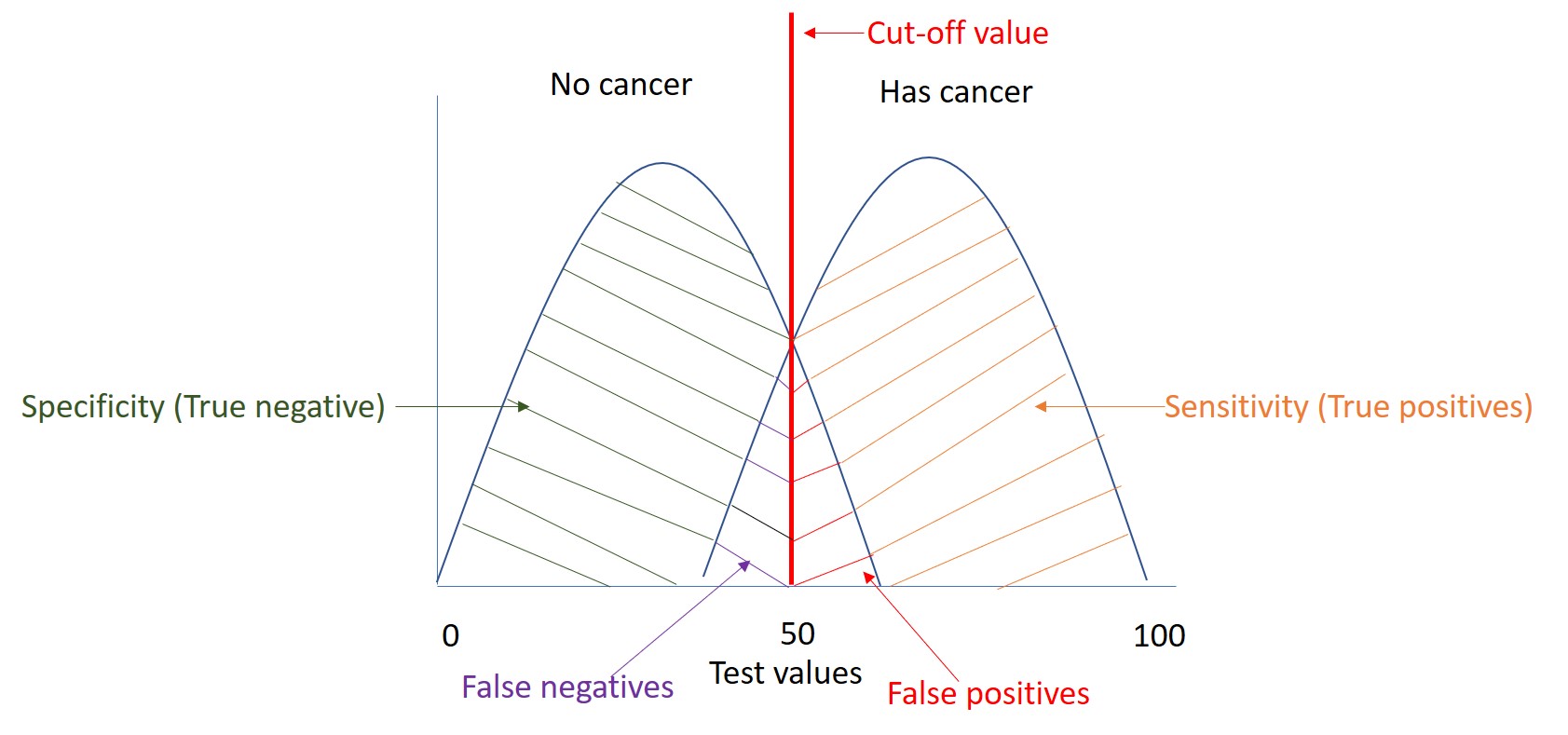
No screening test is perfect (100% specific and 100% sensitive). There is always a trade-off between the sensitivity and specificity of a given screening test as illustrated in the Figure. A test which is highly sensitive will pick up more cases with the disease (orange area in the diagram) at the cost of identifying some people as having the disease when they do not. These tests are referred to as false positives (positive tests in people without disease, red area). In contrast, a test that has a high specificity will inevitably lead to a higher proportion of false negative tests (negative tests in people with disease, area in purple). In the figure our test has a value of 50 (cut-off value), any value above this will be considered positive and negative if it is below this value . If we moved our cut off to 80 then we will increase specificity but decrease sensitivity. In contrast, if we change our test value to 30, we will increase sensitivity but decrease specificity.
Screening tests must be effective, safe, well tolerated with low rates of false positive and false negative results.
Deciding when to call a screening test positive
In practice the definitions of when a test is considered positive and when it is considered negative (cut-off values) are based on several factors such as:
- How invasive the diagnostic test is. For example, if a person screened positive for melanoma cancer and went onto have a biopsy of the skin (as the diagnostic test) this wouldn’t be as invasive as if that same person had a positive prostate screening blood test and went on to have a biopsy. One could argue that the melanoma screening test could be less specific than the prostate screening test because a prostate biopsy is more invasive and has a greater risk of harm than a skin biopsy.
- Potential harms of the diagnostic test or the treatment following a positive screening test. Treatment for cervical disease can increase the risk of having a subsequent preterm birth. So, having a very sensitive test to lesions that may or may not turn into cancers if left untreated, could lead to more women (particularly those who are of childbearing age) being treated for lesions that would have cleared without intervention and increasing the risk of preterm births.
- Health service capacity to see and treat those who test positive. Screening for bowel cancer using the fecal immunochemical test (FIT) allows for a continuous measurement of how much blood is in the stools, hence it is theoretically possible to adjust the sensitivity and specificity of this test. However, those found to be positive on FIT are invited for a colonoscopy and this investigation requires substantial healthcare capacity. Therefore, cut-off values are usually set within the context of available resources
The views expressed are those of the author. Posting of the blog does not signify that the Cancer Prevention Group endorse those views or opinions.


Pingback: Screening iconoclasm series: Don’t be overly sensitive about sensitivity – Cancer Prevention Group Blog
Pingback: Isolines and Rabbit Holes: Adventures in ROC Space – Cancer Prevention Group Blog
Pingback: From theory to practice: HPV testing in cervical screening really does work! – Cancer Prevention Group Blog
Pingback: To tell or not to tell – what should we do? – Cancer Prevention Group Blog